EO sterilization processes are complex and highly regulated
EO is the most widely used gaseous sterilization agent in the world and has been around for nearly 90 years. Today, EO is used to sterilize more than 20 billion medical devices sold in the U.S. every year. There are several regulatory requirements for the EO sterilization process, and Sterigenics maintains collaborative relationships with regulatory agencies.












Sterigenics operates safely and in compliance with regulatory requirements
Sterigenics consistently complies with environmental permits issued for each of its sterilization facilities. Sterigenics has disclosed its EO emissions for decades in federal and state filings – even when not required and when others in the industry did not – and has a track record of implementing leading safety practices to further perform better than regulatory requirements.
The EPA has recently agreed to reconsider the use of the IRIS value for EO in assessing cancer risk for the source category
The State of Texas Commission on Environmental Quality (TCEQ) recently concluded that acceptable EO levels may be more than 2,000 times higher than those suggested by IRIS
IRIS concentration of 0.1 parts per trillion is about the same as:

1
Drop of water within...

200
Olympic size swimming pools

EPA IRIS Level is Far Lower than Normal EO Exposure from Everyday Sources
Measured in ug/m3
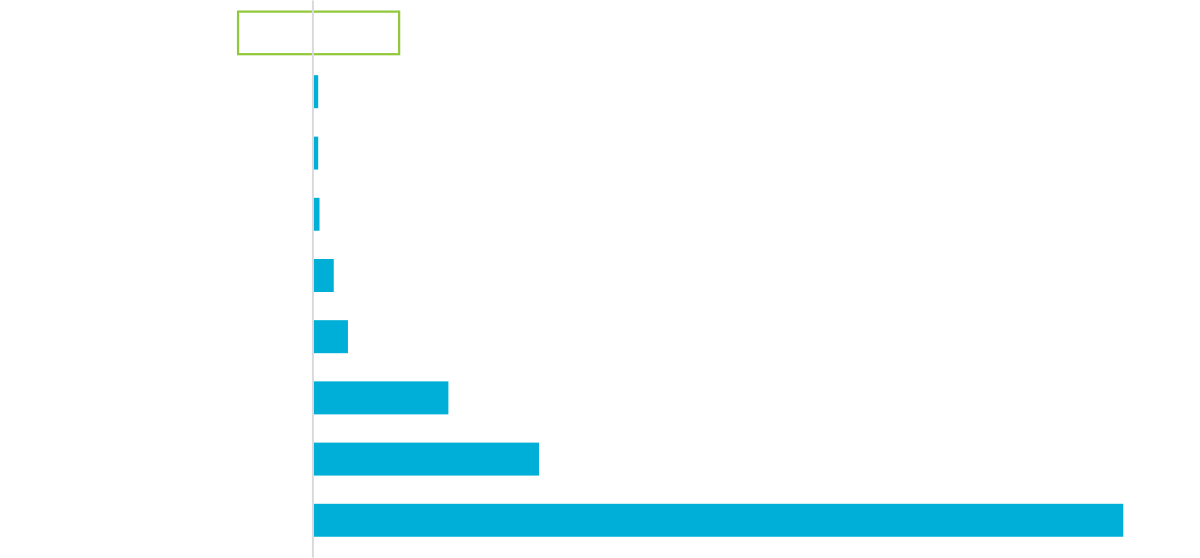
Montrose Air Quality Services Measurements of “Everyday Sources” for Ethylene Oxide, October 16, 2019