EO exists naturally and comes from a variety of sources
Natural Sources


Everyday Sources





EO has a variety of modern uses
EO is primarily used to make other chemicals; and EO, and its derivatives, also help make or supply a variety of products we use every day:








EO exists in the air all around us
Given the variety of sources and modern uses, EO exists at “background levels” across the country.
The average EO level across the U.S.:
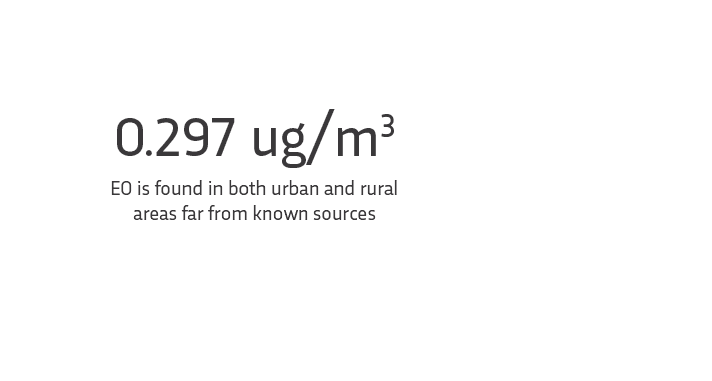
Compared to:
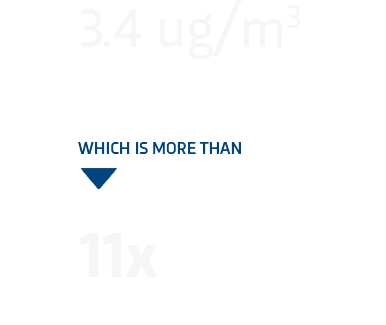
ug/m3 = micrograms per cubic meter
U.S. EPA: Ethylene Oxide Ambient Concentrations at National Air Toxics Trends Stations and Urban Air Toxics Monitoring Program stations October 1, 2018 – March 31, 2019
EO is essential to the U.S. health care system
The U.S. Food & Drug Administration (FDA) has stated repeatedly that EO is essential to the U.S. health care system. Hospitals and patients depend on EO sterilization for critical and lifesaving products. Billions of medical products and devices are used every year, and many must be sterile before use to ensure the safety of patients and health care providers.
>20B
medical devices sold in the U.S. every year are sterilized with EO
~50%
of medical products that require sterilization in the U.S. are sterilized with EO
60%
of essential medical devices used to combat pandemics are sterilized with EO

“Without adequate availability of ethylene oxide sterilization, we anticipate a national shortage of these devices and other critical devices including feeding tube devices used in neonatal intensive care units, drug-eluting cardiac stents, catheters, shunts and other implantable devices. It’s important to note at this time there are no readily available processes or facilities that can serve as viable alternatives to those that use ethylene oxide to sterilize these devices. In short: this method is critical to our health care system and to the continued availability of safe, effective and high-quality medical devices.”

“For many medical devices, sterilization with EO may be the only method that effectively sterilizes and does not damage the device during the sterilization process.”
Examples of medical devices sterilized using EO
 Gowns/Drapes
Gowns/Drapes Surgical Kits
Surgical Kits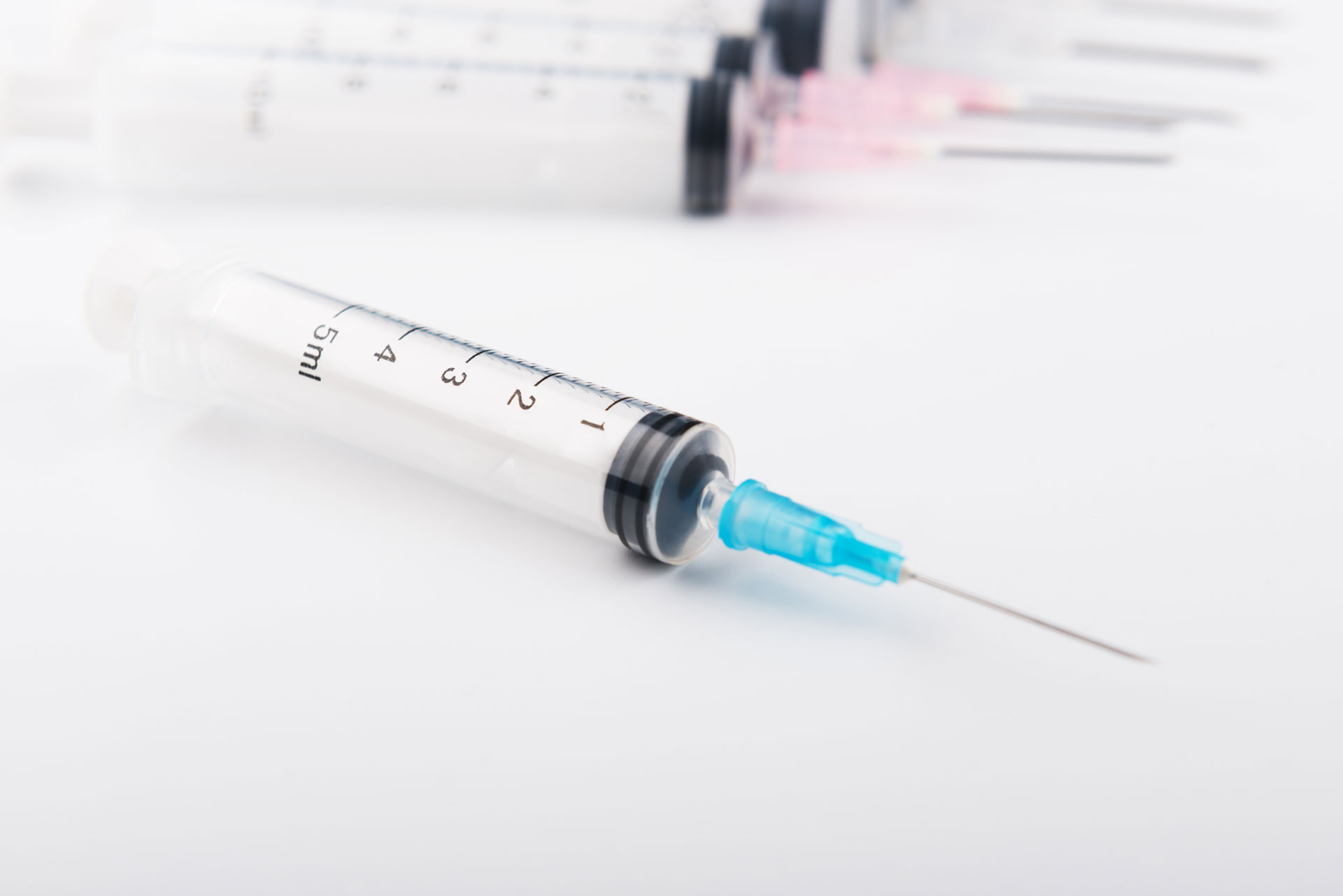 Syringes
Syringes Pacemakers
Pacemakers
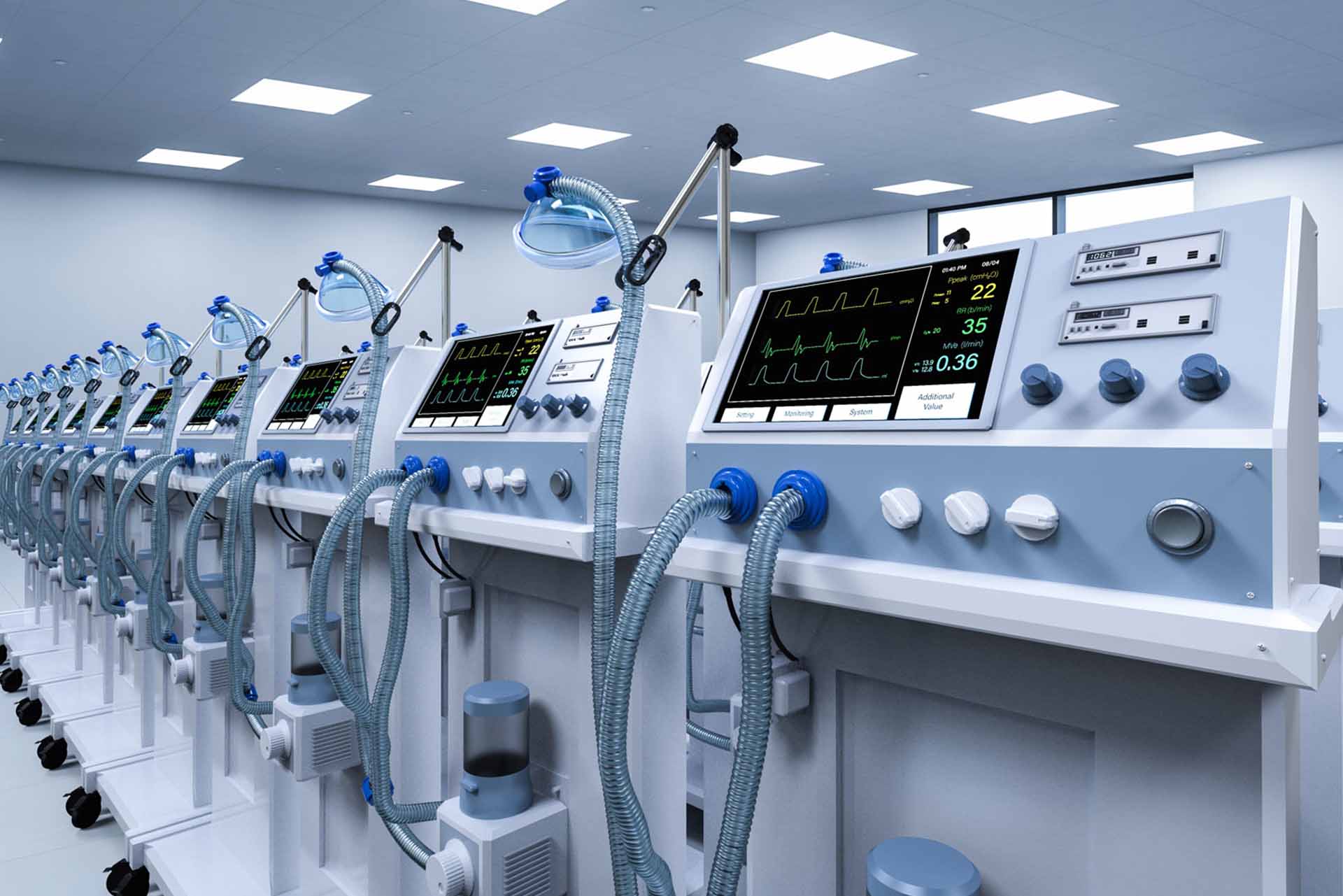 Ventilators
Ventilators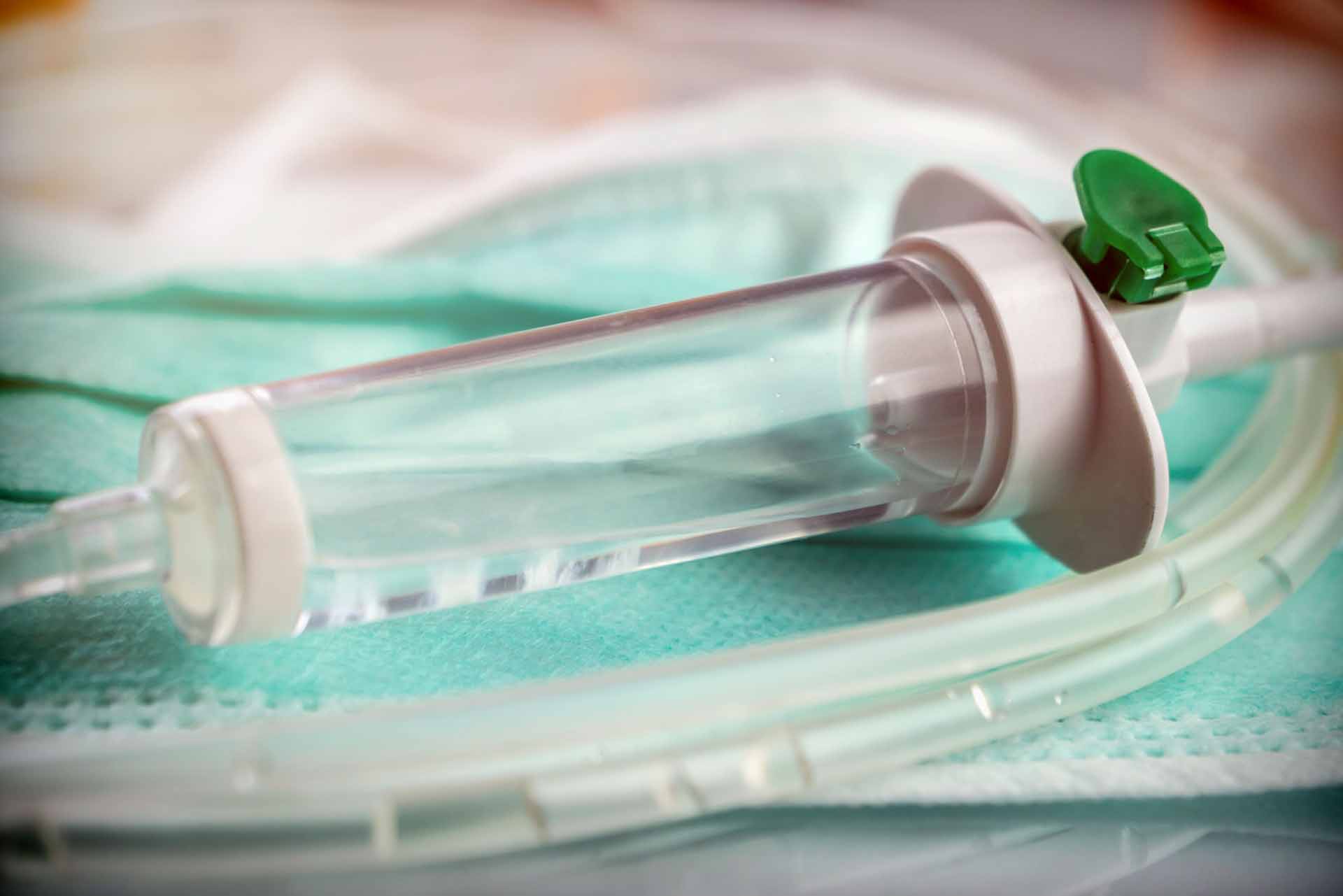 IV Sets
IV Sets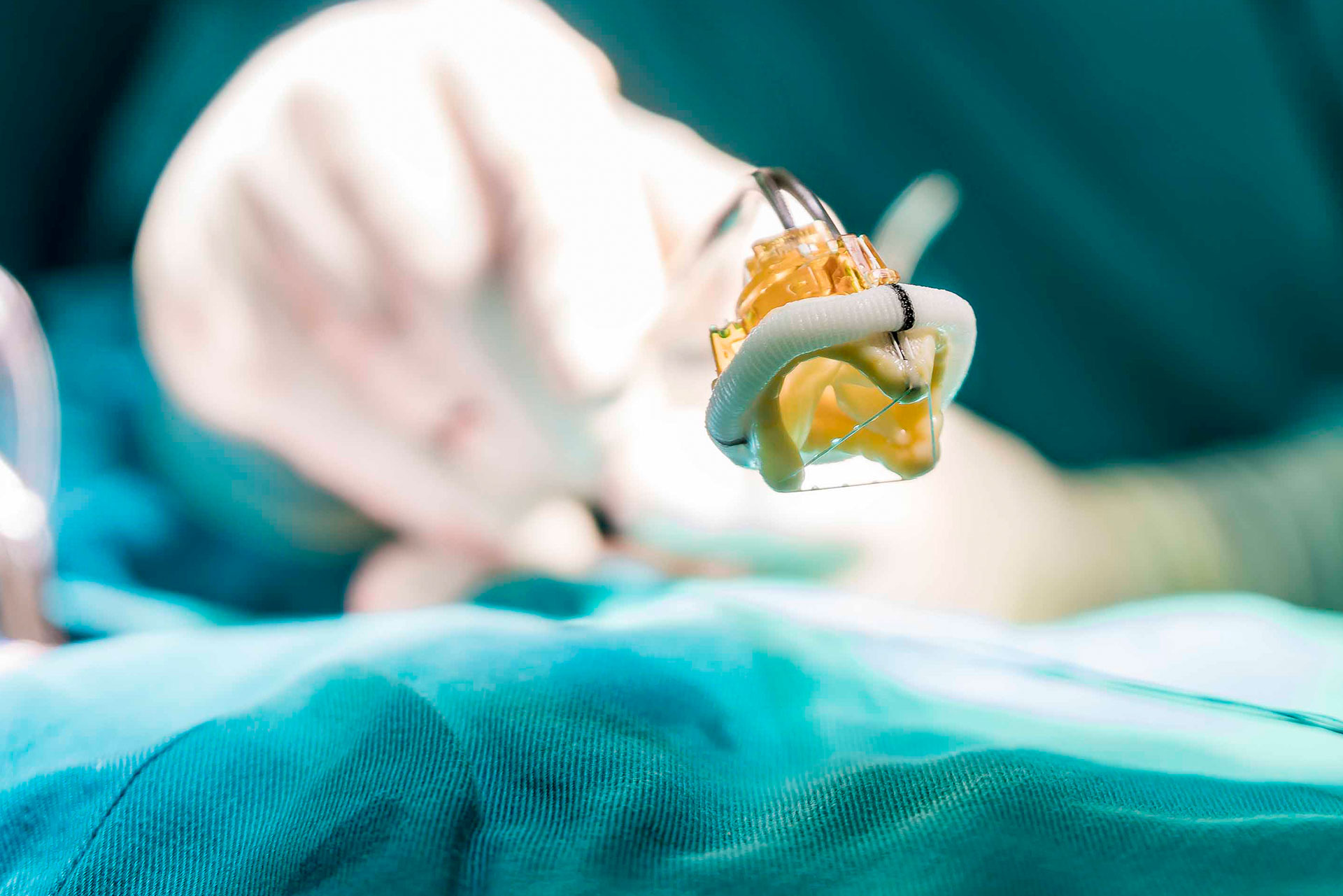 Heart Valves
Heart Valves Sutures
Sutures